111 | Add to Reading ListSource URL: cdn.govexec.com- Date: 2012-05-17 08:34:06
|
|---|
112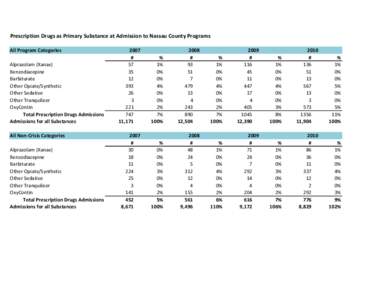 | Add to Reading ListSource URL: heroinprevention.comLanguage: English - Date: 2014-03-11 14:46:53
|
|---|
113 | Add to Reading ListSource URL: cdn.govexec.com- Date: 2012-05-17 08:34:06
|
|---|
114 | Add to Reading ListSource URL: www.accessdata.fda.govLanguage: English - Date: 2011-08-24 15:13:10
|
|---|
115![FDA APPROVED LABELING FOR XANAX XR Attachment to FDA Approval Letter for NDA[removed] FDA APPROVED LABELING FOR XANAX XR Attachment to FDA Approval Letter for NDA[removed]](https://www.pdfsearch.io/img/0b5a864d31bfc320aaa6220136f42635.jpg) | Add to Reading ListSource URL: www.drugbank.caLanguage: English - Date: 2010-02-11 16:34:15
|
|---|
116 | Add to Reading ListSource URL: www.fda.govLanguage: English - Date: 2006-08-03 09:26:30
|
|---|