1 | Add to Reading ListSource URL: apac-asia.comLanguage: English - Date: 2017-05-01 03:53:28
|
|---|
2 | Add to Reading ListSource URL: www.piqur.com- Date: 2016-02-17 03:09:52
|
|---|
3 | Add to Reading ListSource URL: www.piqur.comLanguage: English - Date: 2016-02-17 03:09:52
|
|---|
4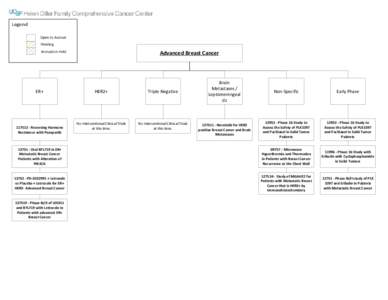 | Add to Reading ListSource URL: cancer.ucsf.eduLanguage: English - Date: 2014-10-06 14:59:53
|
|---|
5 | Add to Reading ListSource URL: www.eisai.comLanguage: English - Date: 2015-01-13 01:46:50
|
|---|
6 | Add to Reading ListSource URL: dvha.vermont.govLanguage: English - Date: 2015-02-20 16:02:44
|
|---|
7 | Add to Reading ListSource URL: www.pbs.gov.auLanguage: English - Date: 2014-10-02 00:13:48
|
|---|
8![Microsoft Word[removed]Eribulin Eisai PSD March 13 final.docx Microsoft Word[removed]Eribulin Eisai PSD March 13 final.docx](https://www.pdfsearch.io/img/634748c785128f92b6ce2951b7aabadc.jpg) | Add to Reading ListSource URL: www.pbs.gov.auLanguage: English - Date: 2015-03-06 00:23:42
|
|---|
9 | Add to Reading ListSource URL: www.accessdata.fda.govLanguage: English - Date: 2014-08-04 14:23:47
|
|---|
10 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|